While large companies and public sector consortiums in the United States, Canada, China and Europe are running at full speed to develop a vaccine grown in genetically modified (GM) tobacco plants, a research group at a Mexican university is working toward the same objective, but with a different and innovative strategy. They are using bioinformatics and computational genetic engineering to identify candidate antigens for a vaccine that can be expressed in tomato plants. Eating the fruit from these plants would then confer immunity against COVID-19.
At the time I write these lines, there are already more than 3.6 million people reportedly infected by the COVID19 pandemic and some 252,000 deaths globally. In the US, which has the world’s highest rate of infection, COVID-19 deaths have surpassed deaths from cancer, coronary heart disease and even influenza/pneumonia in just the few months since the novel coronavirus arrived.
This critical situation has led the entire world to embark on a real race to develop a vaccine that immunizes the population against this new strain of coronavirus, which apparently emerged in the autumn of 2019 in China. So far, more than 100 vaccines are being investigated for COVID-19 by universities, public research centers and especially private companies. Some are already under clinical trial.
The approaches used for their production don’t differ much from the ones classically used in vaccines, where the antigens — a compound of the pathogen used to generate immunity in the patient — can be the inactivated virus, as well as the genetic material or a virus protein, which is grown on a large scale in chicken eggs, mammalian/insect cell tissue or genetically modified microorganisms.
Plants as vaccine biofactories
A lesser-known approach to produce antigens and vaccines on a large scale is the use of plants as biofactories. The plants are genetically modified (Figure 1) to produce, for example, virus-like-particles (VLPs), which are structural proteins of the virus, or “multi-epitope” proteins, where different sequences of an antigen allow us to generate an immunizing and protective response in humans.
The most widely used plant is Nicotiana benthamiana, a close relative of tobacco, due to its biomass, easy laboratory management and rapid growth. But scientists have also worked with other crops, such as lettuce, carrots, potatoes, rice, tomatoes and corn, among others.
At the beginning of 2020, 97 experimental vaccines had been obtained with this methodology, including plant-grown antigens for HIV, polio, hepatitis B, rabies, HPV, cholera and tuberculosis, among other pathogens. Work even has been carried out on the cultivation of compounds against cancer and autoimmune diseases.
Some of the plant-based vaccines that have made it to advanced clinical trials include a flu vaccine developed by Medicago, a Fraunhofer malaria vaccine and ZMapp, a three-monoclonal antibody serum developed by Kentucky Bioprocessing, which has already been used with patients in outbreaks of Ebola from 2014-2015 and 2018-2019 in Africa. All these vaccines were obtained through cultivation of GM tobacco.
Currently, plant-based drugs are already a reality and at least one has entered the market: taliglucerase alfa, an enzyme grown in GM carrots and obtained in bioreactors, which is prescribed as replacement therapy for Gaucher disease.
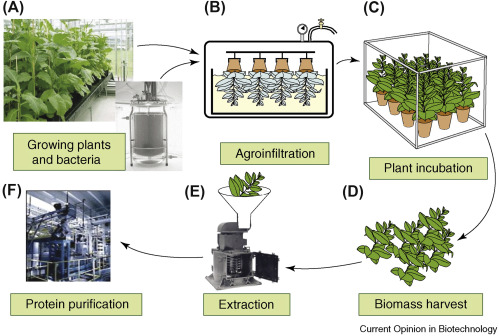
The advantages of vaccines cultivated in plants include the facilitation of their transport and storage without the need for a cold chain, which lowers costs. In addition, there is no need to worry about contamination of toxins and pathogens for humans, a risk that can occur in the production of vaccines in microorganisms or mammalian cultures.
Efforts in COVID-19 from the public and private sectors
Within the COVID-19 vaccine race, the strategy of plant cultivation — also know as biopharming or molecular farming — hasn’t been left out. Two companies already mentioned are working on COVID-19 plant-based antigens by expressing VLPs in GM tobacco. One of them is Medicago, whose CEO claimed the Canadian company would be able to manufacture “10 million doses per month” if its innovative production method and clinical trials obtain US Food and Drug Administration (FDA) approval. On the other hand, the American company Kentucky Bioprocessing is using a fast-growing GM tobacco of its own and publicly stated that it is already conducting preclinical tests and possesses the ability to manufacture up to three million doses per week.
The third private sector research group is an alliance between the US company iBio and China’s Beijing CC-Pharming, which are combining the cultivation of VLPs from COVID-19 and a lichenase carrier immunostimulatory adjuvant in GM tobacco.
Meanwhile, the public sector is not far behind. The University of California, San Diego, is working on an innovative collaborative project between internal research groups to develop a microneedle patch-vaccine that uses proteins grown in GM plants.
On the other hand, the Center for Research in Agricultural Genomics (CRAG) of Spain, will develop antigens for COVID 19 in GM lettuce and tobacco, and the international project NEWCOTIANA, which works on the development of medicines and vaccines in plants with funding from the European Union, has released the complete genetic sequence of Nicotiana benthamiana in order to accelerate the development of a plant-based vaccine. This last work was led by IBMCP (Spain) and Queensland University of Technology (Australia).
What if the vaccine could be “eaten” instead of injected?
Although the plant-based vaccines mentioned above have certain advantages over conventional vaccines, their route of administration continues to be through parenteral injection, the “jab” that can cause so much pain to children. But what if, instead of using GM tobacco and purifying antigens to make an injectable vaccine, we could eat a GM fruit that directly confers immunity?
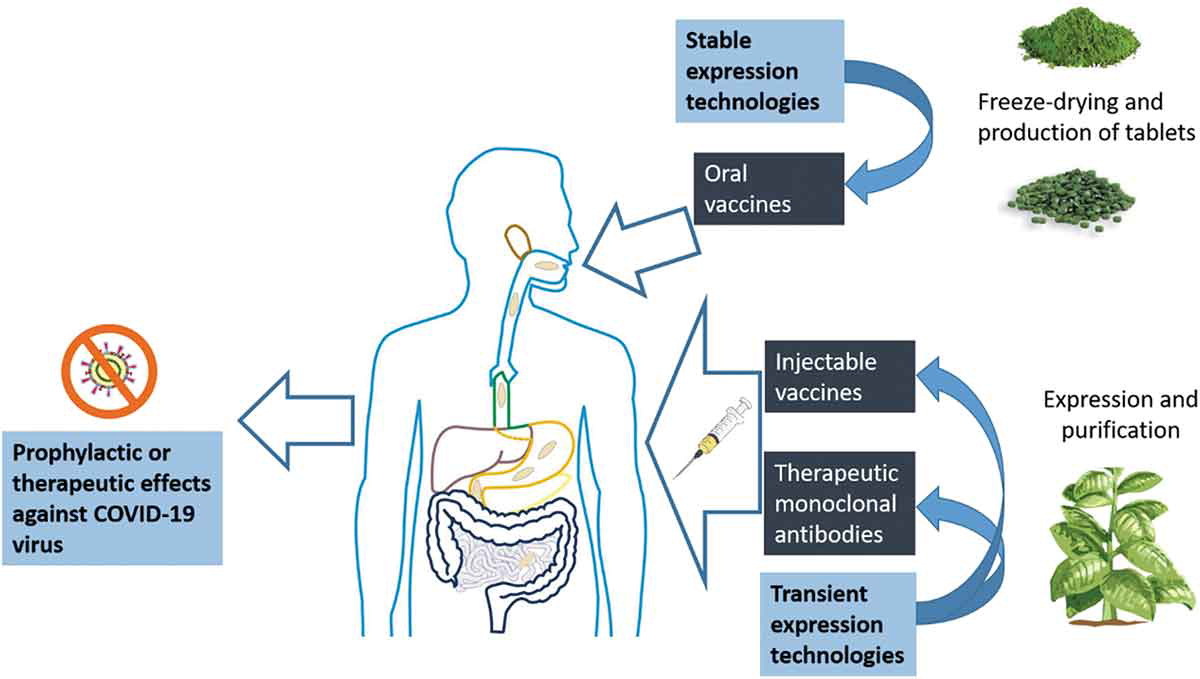
Although something like this is not yet in clinical use, it is not a novelty in experimental terms. Since the 1990s, several research groups have worked on the modification of edible plants and fruits that generate an immune response in the intestinal epithelium of animals after oral intake (Figure 2). Genetically modified crops — still at the experimental, not commercial, level — used to create “edible vaccines” range from potato, tomato, lettuce, papaya, carrot and rice to quinoa, alfalfa, banana and algae. They have focused on hepatitis B, rotavirus, Norwalk virus, malaria, cholera and autoimmune diseases, among others.
Garza’s research
This route was the one chosen by Daniel Garza, a young biotechnologist and entrepreneur with a research stay at the Institute of Biotechnology of the Autonomous University of Nuevo León (UANL) in Mexico, as an approach to developing a vaccine against COVID-19. “The development of an edible vaccine against SARS-CoV-2 has so far been a little-explored alternative, even though the benefits are evident,” Garza said in an interview with the Cornell Alliance for Science. “Under this premise, this problem would be addressed with the focus of developing a fusion protein with the characteristics of a vaccine to be expressed in tomato plants.”
Garza, together with a multidisciplinary group of researchers, use bioinformatics and computational genetic engineering in applying a reverse vaccination strategy. More specifically, by using bioinformatics tools, they identify the antigens most likely to be vaccine candidates to induce an immune response through “in silico” analysis of the pathogen genome.
“The development of vaccines using conventional techniques depends on a large number of biochemical, immunological and microbiological methods that require a large amount of time and that imply higher production costs,” Garza said. “The reverse vaccination strategy offers the possibility of identifying a greater number of proteins for each pathogen and selecting the best candidate vaccine antigens. This allows the development of vaccines that were previously difficult or impossible to manufacture.”
Researchers from Garza’s laboratory have been working with this approach since 2018 to search for new candidate antigens for an Ebola vaccine — research they published at the end of 2019 in the UANL magazine Planta. “The results observed so far allow us to identify new epitopes in the regions of the sequence of the VP40 protein of the Ebola virus with characteristics of immunogenicity, antigenicity, hydrophilicity and accessibility that make them a vaccine candidate,” Garza said.
Once the candidate sequence has been identified, they continue optimizing the nucleotide sequence in the tomato plant and the genetic transformation by Agrobacterium tumefaciens. “The expression in tomato plants with the new identified epitopes allows us to obtain high levels of expression of the recombinant protein” Garza added. In simple terms, prior bioinformatic modeling saves effort and works with antigens that have a high protective response against the pathogen, a useful antigen for the development of a viable and scalable vaccine.
However, due to the contingency and severity of the SARS-CoV-2 outbreak, Garza’s group decided to dedicate its efforts to work on the bioinformatic modeling of a potential vaccine for this pathogen, using the same strategy used against the Ebola virus through the development of an edible tomato as an immunization method.
The only similar work that can be found in the bibliography is the development of a tomato with SARS-CoV antigens, which was responsible for severe acute respiratory syndrome (SARS) in Southeast Asian countries in 2002-2003 and has 70 percent genomic similarity to the pathogen behind the current pandemic. Although mice immunized orally with this transgenic tomato revealed significantly high levels of specific antibodies against SARS-CoV-1, there was no further progress towards clinical phases.
“We are at the analysis stage, using the genomic and proteomic sequences of SARS-CoV-2 and using bioinformatic tools that allow us to identify the antigens most likely to be candidates to induce an immune response,” Garza said in regard to the current status of efforts to develop a vaccine against SARS-CoV-2, the virus that causes COVID-19, in tomato plants.
“The candidate epitopes are selected based on the prediction of their function, such as accessibility and secretion, and then they are cloned, expressed and analyzed to subsequently confirm their cellular location in vitro. The use of animal models will allow us to evaluate their immunogenicity and protective capacity,” Garza added.

As Garza explained, this research is currently in the stage of analysis and identification of potential regions for the development of a vaccine. His research team is now applying its project to a “general call” addressed to Mexican researchers working on the development of drugs against COVID-19 that was issued by the Mexican government, which finances the expenses for a collaboration with the Paul Scherrer Institute of Switzerland. The next phase of the project will be the expression of the candidate antigens in tomato and evaluating their immunogenic and protective capacity in animal models. As the project progresses, links with companies or research centers will be evaluated to bring the candidate vaccine to the clinical phase.
Benefits of an edible vaccine
Beyond eliminating the annoying “prick” of a needle, using fruits or edible plants to immunize people against diseases offers several benefits, including reduced production costs since there is no need for treatment or purification prior to oral administration.
The direct consumption of a raw material — either through the fruit itself or its lyophilized biomass encapsulated in gelatin pills or tablets — is a clear advantage as it reduces the cost of antigen processing and purification, as well as the degradation of antigens in the gastrointestinal tract due to the protective role of plant cells within the stomach.
Additionally, the expression of the antigen in the seeds allows maintenance and stability for longer periods. Edible vaccines can also produce complex multimeric proteins that cannot be expressed by microbial systems and are a safe and effective method of vaccination.
The fact that edible vaccine formulations don’t require antigen purification probably would be the main factor contributing to their low-cost, which is necessary to achieve broad vaccination coverage in developing and low- income countries.
Statistics show, for example, that only 40 acres would be required to produce all the annual hepatitis B vaccines for the entire population of China, and only about 200 acres to produce edible vaccines for all children globally. The final objective of this type of technology would be to deliver not only “vaccines” but also real “medicinal foods” — not in the alternative or marketing sense, but in a literally curative sense — through plants and fruits that reinforce overall health and protect the immune system against pathogens, cancer or autoimmune diseases. This is especially important in underdeveloped countries, where it is difficult to obtain treatments or procedures that require complex equipment and conventional vaccines are hard to store and transport.
Pending challenges
Regulatory and biosafety obstacles, rather than technical and experimental constraints, could delay the arrival of edible vaccines to our tables and hospitals, especially in the countries most in need.
Although many countries on all continents develop, or have developed, GM crops on an experimental level, only 26 nations currently have regulations implemented for their commercial use. The fact that so many countries lack legislation, or use backward and cumbersome regulatory frameworks, such as the one employed by the European Union, could increase the final cost of bringing the edible vaccine from the laboratory to the market, making it difficult for small and medium-sized companies or public institutions to develop this technology.
In the case of Mexico, where they are already working on the development of an edible vaccine in tomato plants against COVID-19, local scientists are dealing with difficult times under the mandate of a president who has repeatedly declared himself against the use of GM crops and who appointed a scientist famous for being a staunch GM opponent as director of CONACYT, the government entity that regulates the national science budget. Nor can we forget the recent problem of Mexican cotton growers, who aren’t receiving new permits for the cultivation of GMOs by the current government..
“Given the current contingency situation for the COVID-19 we are experiencing, it will undoubtedly make us rethink the legislation of the GMOs that apply not only in Mexico but in Latin America,” Garza noted. “What is currently happening allows us to rethink whether we are really capable as countries of being able to face a pandemic of such magnitude without using the full potential that GMOs offer us for the development of vaccines, especially for developing countries … The benefits of biotechnology must be shown to society not as an evil, but as an effective solution to many of the problems that we currently have in the region.”
Paradoxically, if the investigation into this promising edible vaccine — created in the Mexican public sector — progresses successfully, it is highly likely that its development towards the clinical phase and productive escalation would move north, to the US or Canada, where companies are already working in molecular pharming aimed at COVID-19 and have the world’s most agile GMO regulatory frameworks. This could happen despite high-level research centers and top scientist working in agricultural biotechnology in Mexico, such as CIMMYT, CINVESTAV and INIFAP, as well as local universities with biopharming capabilities.
Facing the background of regulatory agencies that haven’t developed regulations for plant-based pharmaceutical compounds, and the imminent arrival of some vaccine of plant origin for COVID-19, the Mexican researcher Sergio Rosales Mendoza, who investigates recombinant vaccines in plants and algae in the Autonomous University of San Luis de Potosi (UASLP), concluded an editorial in the journal Expert Opinion on Biological Therapy with key questions:
“Will regulatory agencies display flexibility to validate and approve anti-SARS-CoV-2 virus plant-made biopharmaceuticals (e.g. by adapting/simplifying the regulatory requirements for this specific technology)? Will regulatory agencies accelerate the evaluation process of plant-made biopharmaceuticals against SARS-CoV-2 virus? Will the existing clinical trials of plant-made vaccines against influenza (with encouraging findings) and the enzyme already approved make the approval pathway smoother? Will the developing and low-income countries ultimately benefit from the plant-based technologies in the fight against COVID-19?”
A root problem for edible vaccines is the popular misconception still ingrained in many people that GM crops “are harmful to health or the environment,” despite thousands of studies and reviews, public statements from more than 250 scientific/technical institutions that corroborate its safety and more than two decades of consuming GM crops with no reported adverse effects. A central challenge is to continue delivering this information, especially to the public and law-makers in developing countries, at pivotal moments, such as when we see that new “plant-based” technologies are making their niche in other fields, such as revolutionary lab-meat.
Will we tell our grandchildren and great-grandchildren stories from the past of painful injections given to us when we were children? Will they live in a future where a couple of lyophilized carrot and lettuce capsules will immunize them against all the killer pathogens of the 19th, 20th and 21st centuries? Or will a delicious vaccine-tomato salad be enough to protect them against some new virulent strain that doesn’t yet exist?
Perhaps this race against time and the urgency of finding a viable vaccine for COVID-19 will enable GM plants to save millions of lives and clear their reputations, unfairly stained and demonized by fear and disinformation, once and for all.
Recommended references
Rosales-Mendoza, S.; Márquez-Escobar, V.A.; González-Ortega, O.; Nieto-Gómez, R.; Arévalo-Villalobos, J.I. 2020. What Does Plant-Based Vaccine Technology Offer to the Fight against COVID-19? Vaccines, 8, 183.
Capell T.: Twyman, R.; Armario-Najera, V.; K.-C. Ma, J.; Schillberg, S.; Christou, P. 2020. Potential Applications of Plant Biotechnology against SARS-CoV-2. Trend in Plant Science.
Balke, I., & Zeltins, A. 2020. Recent Advances in the Use of Plant Virus-Like Particles as Vaccines. Viruses, 12(3), 270.
Kurup, V. M., & Thomas, J. 2020. Edible Vaccines: Promises and Challenges. Molecular biotechnology, 62(2), 79–90.
Concha C, Canas R, Macuer J, Torres MJ, Herrada AA, Jamett F, Ibanez C. 2017. Disease prevention: an opportunity to expand edible plant-based vaccines? Vaccines (Basel) 5:14.
Pogrebnyak, N.; Golovkin, M.; Andrianov, V.; Spitsin, S.; Smirnov, Y.; Egolf, R.; Koprowski, H. 2005. Severe acute respiratory syndrome (SARS) S protein production in plants: Development of recombinant vaccine. Proc. Natl. Acad. Sci. USA, 102, 9062–9067.
