In the past 50 years, three major discoveries have fueled the field of genetic engineering. Together, they ve revolutionized medicine and agriculture. Individually, they ve sparked controversy.
Genetic engineering: the first major discovery
It s the late 1960s. Somewhere between the summer of love and the last moon landing, scientists discovered something extraordinary about bacteria. To defend themselves from viruses, bacteria evolved molecular scissors called restriction enzymes.
Restriction enzymes recognize and cut specific patterns of DNA sequences. These patterns are common, but they don t show up in the bacteria s own genes. Invading DNA that makes its way into the bacteria gets cut by restriction enzymes and disarmed.
Using restriction enzymes, scientists can cut and paste together DNA from different species. For example, by cutting and pasting the gene for human insulin into bacteria, we can use the bacteria as biofactories to produce insulin for diabetic patients.
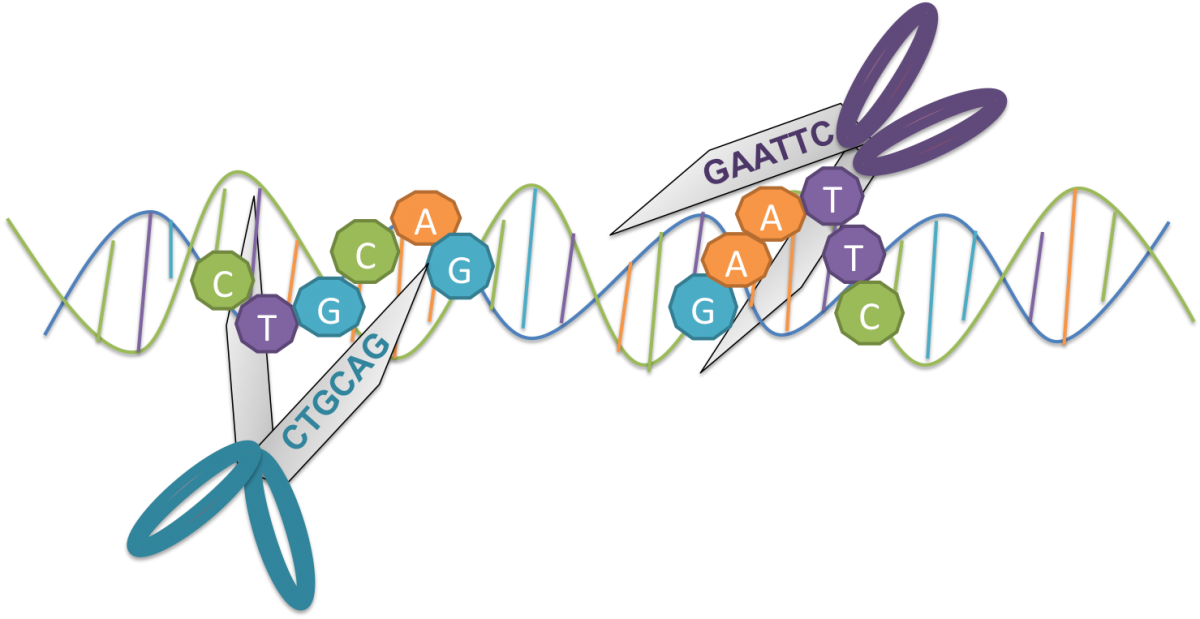
Restriction Enzymes are like molecular scissors that cut specific sequences of DNA. Scientists use restriction enzymes to cut and paste DNA together.
Genetic engineering: applications in agriculture
Flash forward to the early 1980s. Before the war on drugs or the Challenger disaster, scientists discovered something unusual about certain soil bacteria. When these Agrobacteria infect a plant, they cut and paste a small package of DNA into the plant s genome. The package tells the plant cells to divide and make food for the bacteria.
Scientists can employ Agrobacteria to deliver packages of their own. Using restriction enzymes, they replace some of the Agrobacterium s own genes with a gene for a useful trait, such as resistance to insects or pathogens. The Agrobacterium then delivers the gene into a crop plant.
Genetic engineering reinvented: the CRISPR revolution
Then, in 2011, the same year OMG and LOL were added to the dictionary, CRISPR entered the scene. The acronym is a mouthful, but what it really means is that bacteria have an immune system that can learn.
Bacteria store a library of leftover DNA from previous invaders in repeating patterns. This legacy DNA produces a molecular message called RNA, which interacts with a specialized protein. This protein, called Cas-9, cuts invading DNA sequences that match the RNA. This system, called CRISPR-Cas9, allows bacteria to remember and disarm potential threats.
Scientists can use the cut and paste mechanisms of CRISPR-Cas9 to edit genes in all kinds of organisms very precisely.
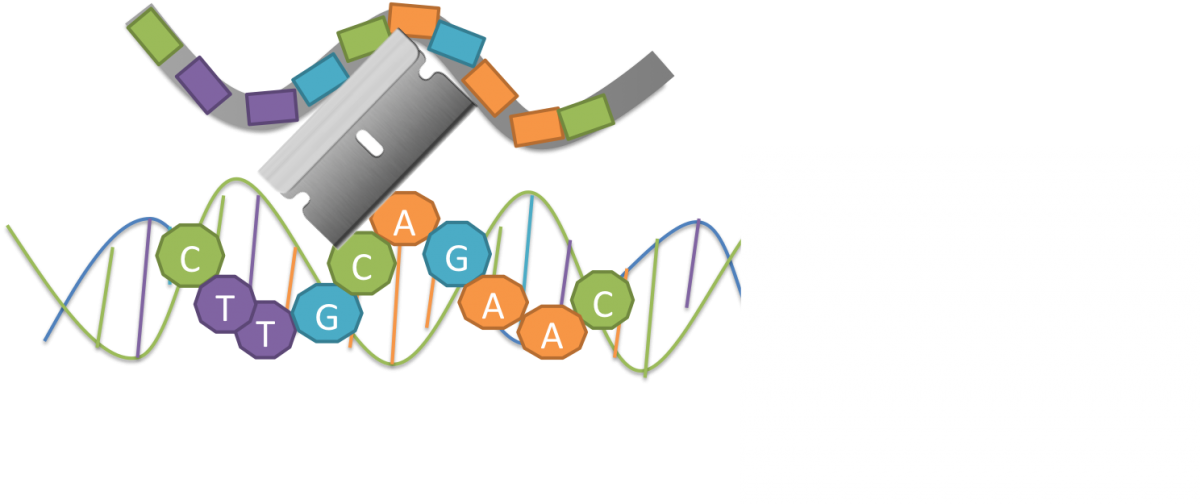
The Cas9 enzyme is like a molecular scalpel that s guided to a very specific DNA sequence by a molecular messenger called RNA. Scientists can use this CRISPR-Cas9 system to make extremely precise edits.
Genetic engineering: prospects and protests
Each of these discoveries restriction enzymes, agrobacteria and CRISPR was stumbled upon somewhat by accident. Studying how bacteria defend themselves has helped us defend ourselves from pathogens and diseases, keep food prices low and develop superior products. But before these tools could be put to work, we had to decide how to use them.
When restriction enzymes were first discovered, scientists thought carefully about the ethics of genetic engineering. In 1975, a small contingent of experts, journalists and lay people gathered at the famous Asilomar conference to discuss what to do with this technology. After four days of heated debates, the participants issued a statement recommending cautions and safeguards. This statement informed the official guidelines issued later by the National Institute of Health.
Although insulin-producing bacteria are literally genetically modified organisms, the term GMO didn t widely circulate until two decades after the Asilomar conference. GMO-mania erupted when scientists began inserting foreign genes into plants.
The vigorous resistance to engineered plants in particular is very puzzling. Few people protest using biofactories to produce medicine. Other foods, such as cheese produced via genetic engineering, have also met with little friction. Yet there are whole organizations committed to protesting GMO crops.
People disagree about whether a plant or animal engineered using CRISPR should be considered a GMO. Historically, GMO status has only applied to transgenic organisms: those containing a gene that was not inserted by traditional breeding.
CRISPR can be used to cut and paste a whole gene into a plant just like restriction enzymes and Agrobacteria. The resulting transgenic plant would certainly be considered a GMO.
On the other hand, CRISPR can be used to make changes that are so small and subtle it would be impossible to tell whether they occurred naturally. For example, if CRISPR was used to replace a single A with a G, this change would be indistinguishable from a natural A to G mutation.
Clearly, genetic engineering is a complex issue. There are many ways to do it, and the line between the different methods can be blurry. The potential applications of genetic engineering are even more diverse.
When stakeholders first gathered at Asilomar to discuss genetic engineering, their debates focused on applications and whether the resulting products could be dangerous. Most scientists agree that debates today should also focus on specific applications of genetic engineering, not the process itself.
After all, the process is not new. Bacteria have been genetically engineering themselves and their neighbors for eons. We only caught on a few decades ago.
